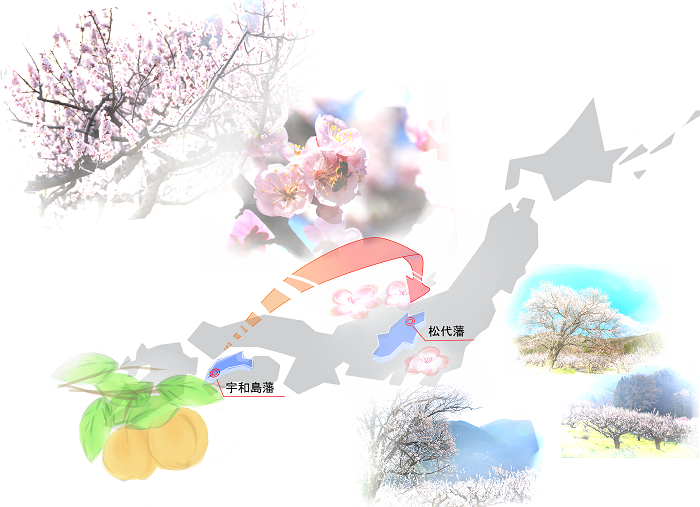
it started from an “Apricot”
 |
 |
 |
Timeline list
・Misao Tomiyama, a representative employee and partner of Kotobuki Shokai, a limited partnership established in 1931, receives a license from Nagano Prefecture to manufacture and sell pharmaceuticals at the company’s Toyono Plant in Nagano City and establishes Kotobuki Seiyakusho.
The limited partnership, Kotobuki Shokai, had produced apricot jam, and produced apricot kernel water from apricot seeds, which were mostly waste1).
The company also manufactures and sells medicines derived from crude drugs produced in Nagano Prefecture (Senega syrup, lotus extract, etc.).
By cultivating herbal medicines in Nagano Prefecture, the company will also contribute to the preservation of the natural environment and the creation of job opportunities in Nagano Prefecture2).
Both 1) and 2) are based on the SDGs.
Apricot kernel water
・Kotobuki Pharmaceutical Co., Ltd. is registered as a corporation (capital of 300,000 yen).
・Misao Tomiyama took office as president.

・Moved the head office to 6351, Sakaki, Sakaki-machi.
・Opened Osaka Office.
・Began production and marketing of a synthetic drug, buformin hydrochloride and triamterene.
・Begins production and marketing of clofibrate, the first domestically produced clofibrate.
・Begins production and marketing of protoporphyrin disodium and exports to Taiwan, South Korea and other overseas markets.
・20th anniversary of foundation.
・Launched Gastritis, Gastric ulcer, duodenal ulcer therapeutic agent MARZULENE-S GRANULES in Japan.
Advertisement at the time of release
Completion of the first stage construction of Research Laboratory.



Panoramic view of the head office
・Completion of the second stage construction of Research Laboratory.
・Introduced high-speed automatic continuous packaging machine and continuous granulation dryer.
・Completion of research facilities of Radio Isotope.
・Our research paper published for the first time in Chemical & Pharmaceutical Bulletin.
・Founding 40th anniversary commemorative magazine “Housu” published.

・Our research paper published for the first time in Journal of Medicinal Chemistry.

・Additional set of drug substance manufacturing equipment in the south area of the second drug substance factory.
・Completion of the second pharmacy plant based on GMP.
・Research paper published for the first time in Bioorganic & Medicinal Chemistry Letters, a journal of the Elsevier’s.
・Kotobuki Pharmaceutical was selected as one of the referees for submitted papers in Elsevier’s “Bioorganic & Medicinal Chemistry Letters.”
・Launched Gastritis, Gastric ulcer, duodenal ulcer therapeutic agent MARZULENE-S GRANULES in India.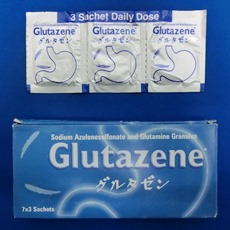
・Launched new Gastric ulcer therapeutic agent AZULOXA GRANULES (Egualen Sodium) in Japan.
・Introduced external lubricant spray device.
・Launched Gastritis, Gastric ulcer, duodenal ulcer therapeutic agent MARZULENE ES TABLETS (Dosage form addition) in Japan.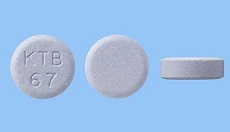



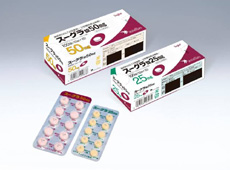
SGLT2 Inhibitor for Treatment of Type 2 Diabetes(Joint development with Astellas Pharma Inc.), in Japan.
Selective SGLT2 Inhibitor “Suglat Tablets 25mg, 50mg (Suglat, Ipragliflozin L-Proline)”
Drug combining selective DPP-4 inhibitor and selective SGLT2 inhibitor “SUJANU Combination Tablets (SUJANU, Sitagliptin phosphate hydrate, Ipragliflozin L-Proline)”
・Approved for manufacturing and sales in Japan, and the drug price is listed and released.
FLT3 Inhibitor “XOSPATA 40mg Tablets (XOSPATA, Gilteritinib)”
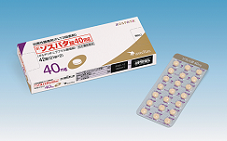
・Relocation of Tokyo Office.
・Research on Kawara OD Tablets®, which uses our unique formulation technology, was selected for the 2021 Nagano Prefecture Pharmacists Association Research Grant 21.
・FY2021 “Nagano Prefecture Pharmacists Association Research Grant 21: Voices of Joy from Award Winners (Representative Director and President Yasushi Tomiyama)” was published in the Nagano Prefecture Pharmaceutical Journal “Rindo”.
・Released “Kawara OD Tablet” in Japan.